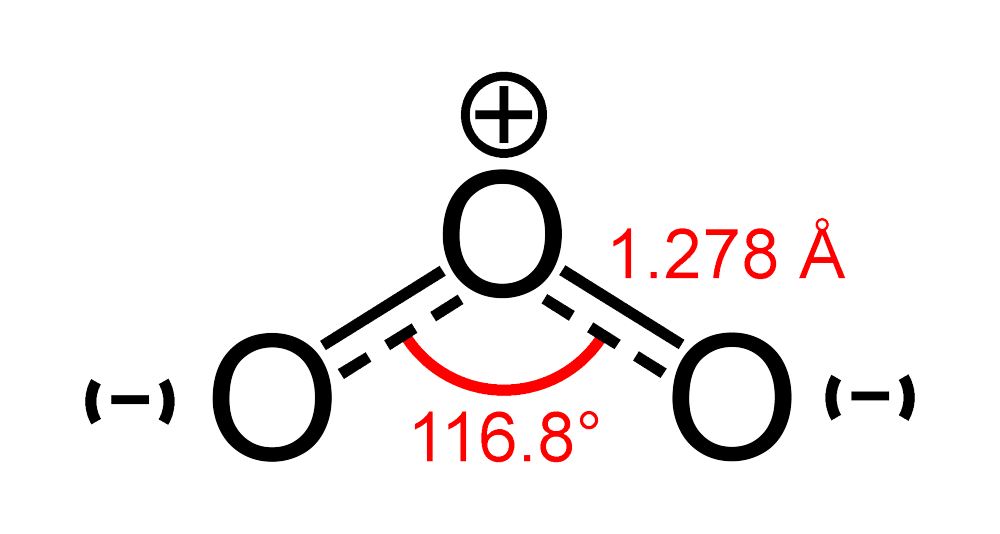
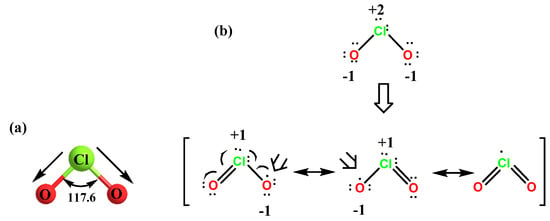
An Integrative Synthetic, Spectroscopic, and Computational Study of the Free Radical Chlorine Dioxide and its Interactions with

Popelle The molecule which has zero dipole moment is (1) CH2Cl2 (2) BF3 (3) NF3 (4) CIOZ 171:of the following model best doorbooth

Popelle The molecule which has zero dipole moment is (1) CH2Cl2 (2) BF3 (3) NF3 (4) CIOZ 171:of the following model best doorbooth

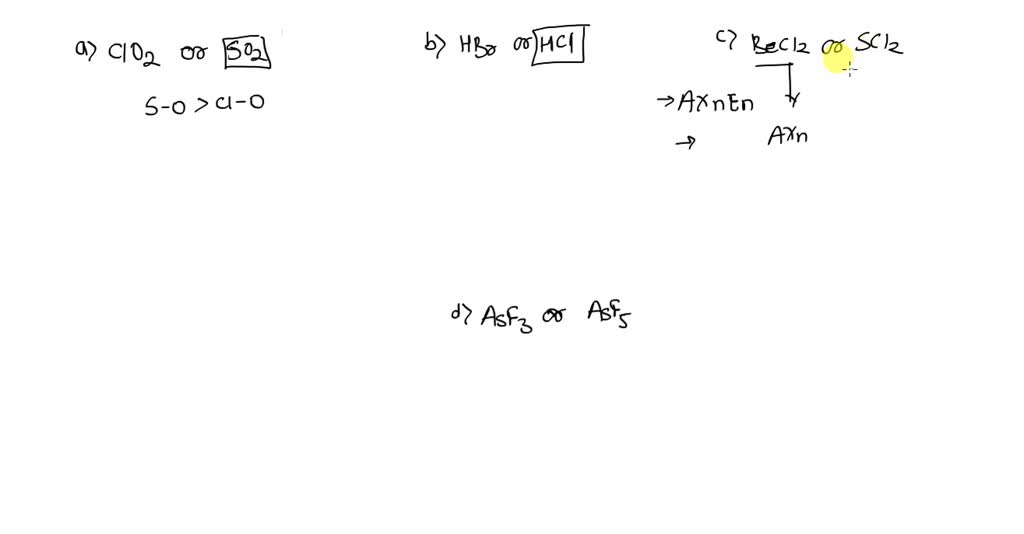
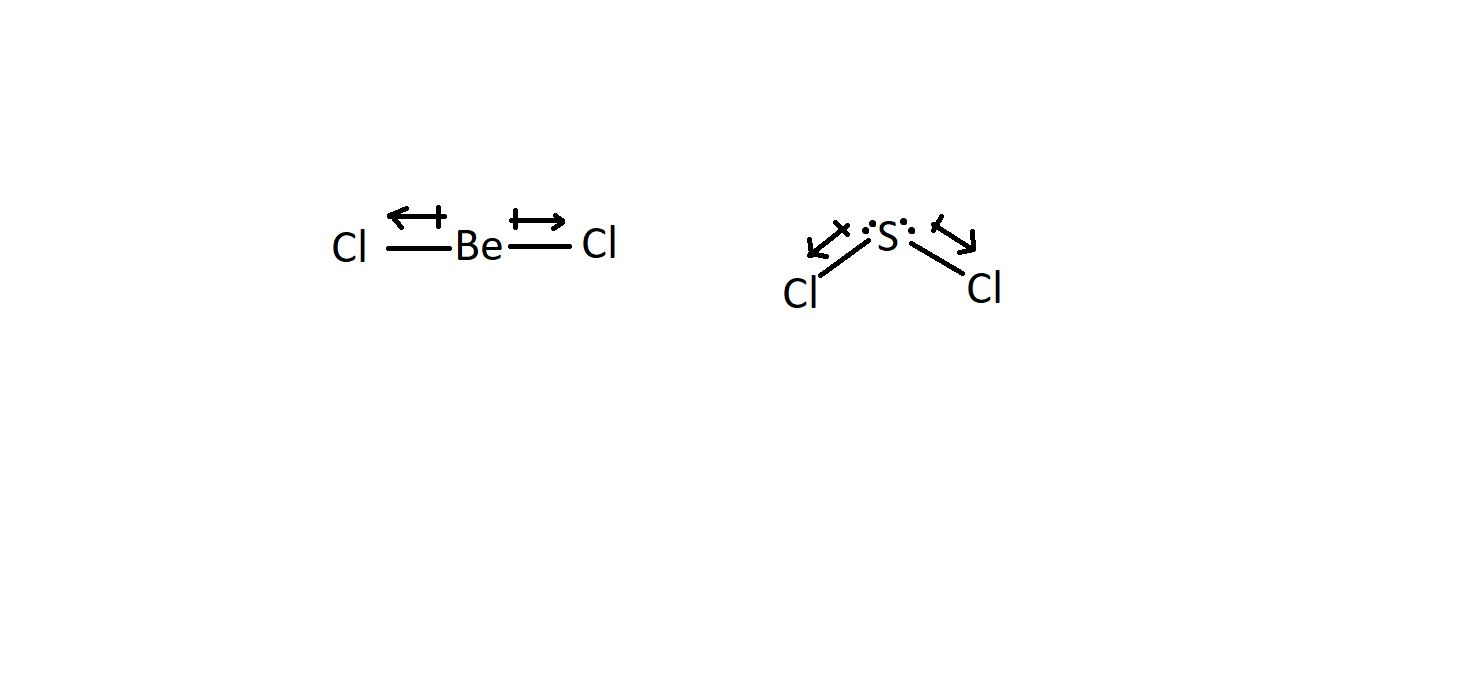
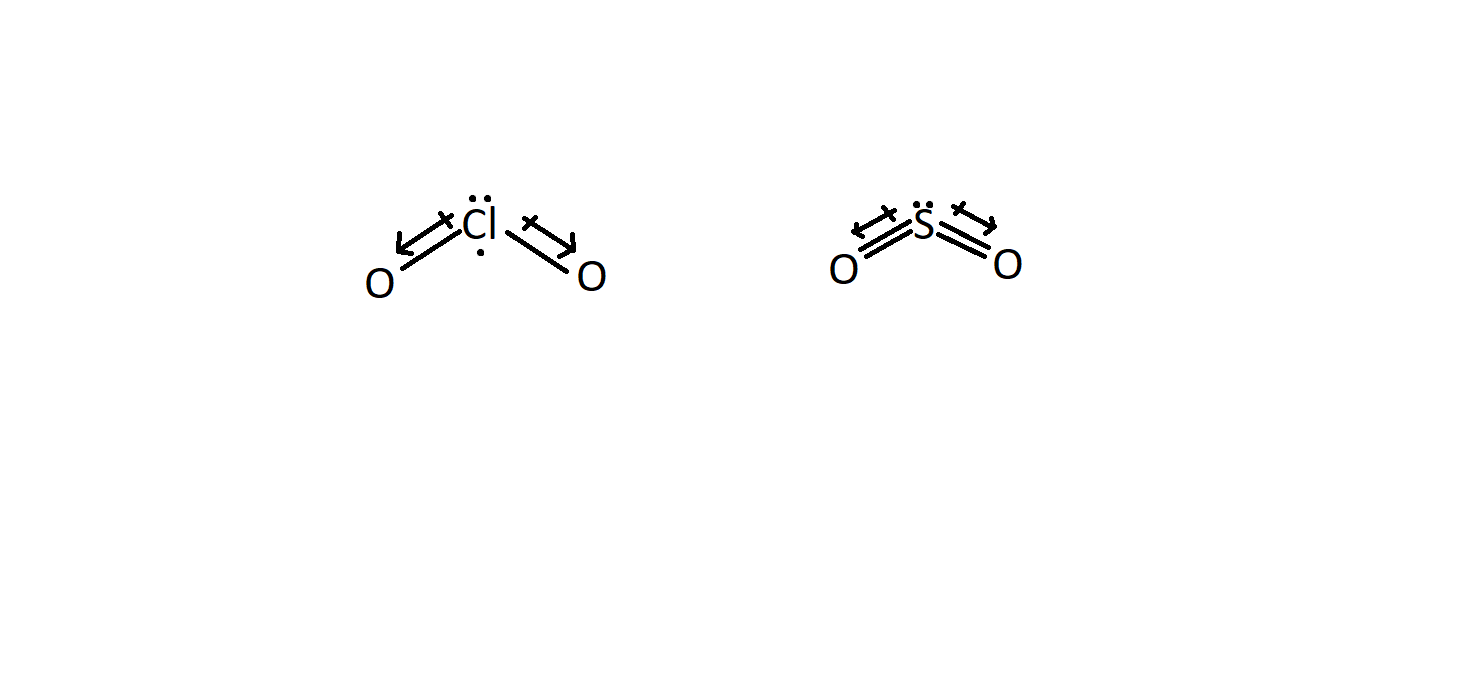
SOLVED: Which molecule in each pair has the greater dipole moment? Give the reason for your choice. (a) ClO2 or SO2 (b) HBr or HCl (c) BeCl2 or SCl2 (d) AsF3 or
An Integrative Synthetic, Spectroscopic, and Computational Study of the Free Radical Chlorine Dioxide and its Interactions with
An Integrative Synthetic, Spectroscopic, and Computational Study of the Free Radical Chlorine Dioxide and its Interactions with