
which of the following pairs have dipole moments (1) BF3 and NCl3 (2) H2O and BeF2 (3) C2O - Chemistry - Chemical Bonding and Molecular Structure - 9666489 | Meritnation.com

Which have a molecular dipole moment? (Select all that apply.) 1. BF3 2. SF4 3. BrF3 4. NF3 5. CF4 | Homework.Study.com

Which have a molecular dipole moment? (Select all that apply.) 1. BF3 2. SF4 3. BrF3 4. NF3 5. CF4 | Homework.Study.com
Define dipole moment? Comment on structure & dipole moment of CO2, BF3. - Sarthaks eConnect | Largest Online Education Community

Question 17 Give reason the following: @ Dipole moment of BF3 is zero but ammonia has a dipole moment. (6) Cuci is covalent than Naci. C) LiCl is covalent than NaCl. (
Dipole moment in case of BF3 is zero. Explain. - Sarthaks eConnect | Largest Online Education Community
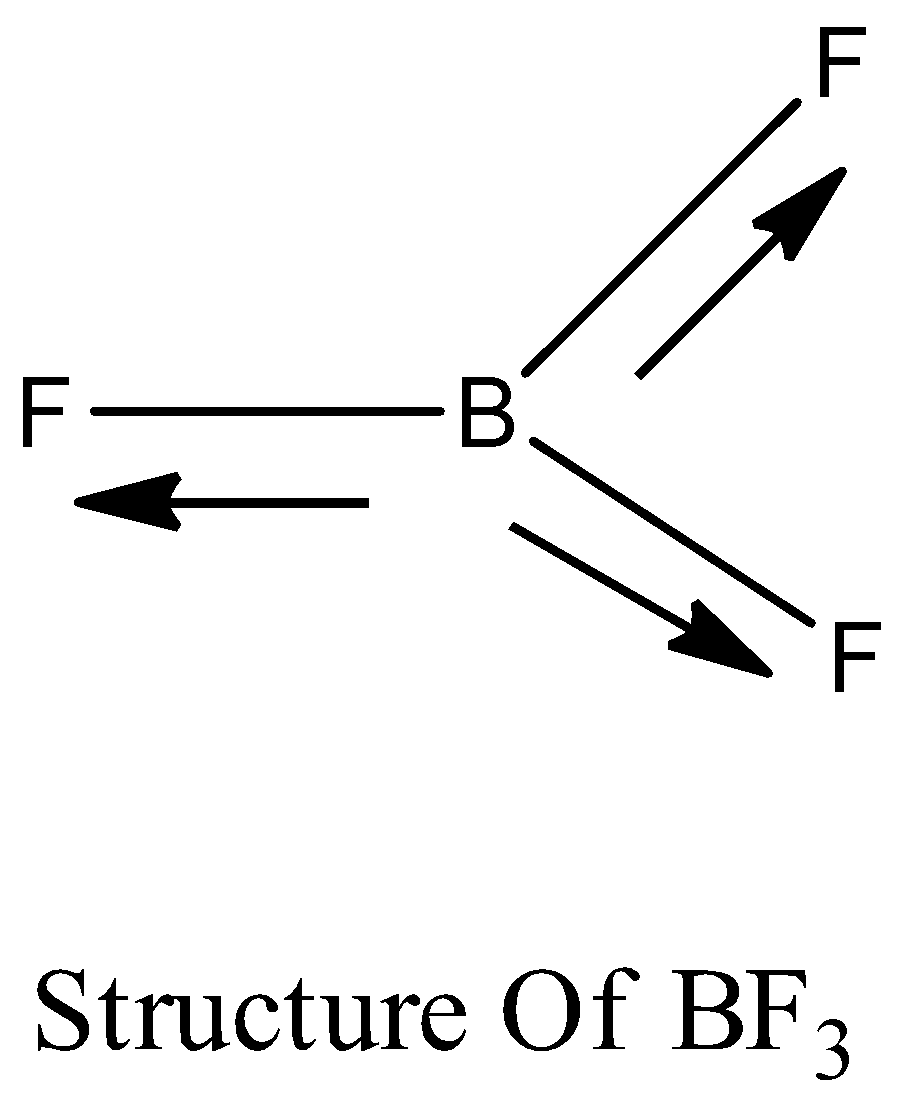
Boron trifluoride $(B{F_3})$ has no dipole moment $(\\mu = 0D)$. Explain how this observation confirms the geometry of $B{F_3}$ predicted by VSEPR theory.

BF3 and NF3 both are covalent compounds but NF3 is polar whereas BF3 is non - polar. This is because :
Which out of the following pairs has dipole moment and why ? nbsp; i] BF3 AND NF3 nbsp; ii] CO2 AND H2O






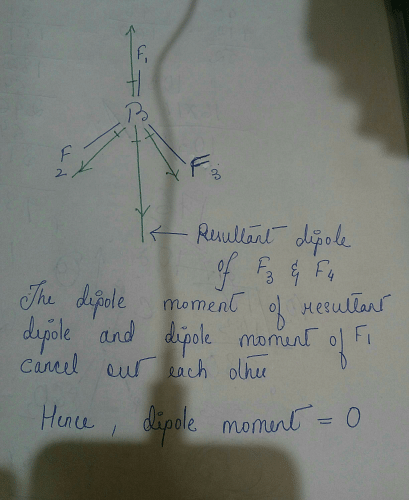

![Odia] Among C Cl4, BF3, NH3 and CO2, Which one has net dipole moment Odia] Among C Cl4, BF3, NH3 and CO2, Which one has net dipole moment](https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/11812254.webp)