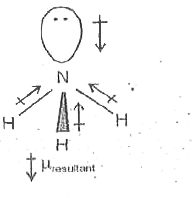
Can you explain why NH3 has such a large dipole moment compared with NF3? Show work. | Homework.Study.com

Is NH3 polar Or Nonpolar? - nh3 Intermolecular Forces, nh3 charge, nh3 bond angle, detailed facts? - chemwhite.com

Ma'am, can you also check this order for decreasing dipole moment- NH3 NF3 NCl3 NBr3 - Chemistry - - 15701287 | Meritnation.com

Effect of on dipole moments of CO, O3, NH3 molecules Dipole moment |P e... | Download Scientific Diagram
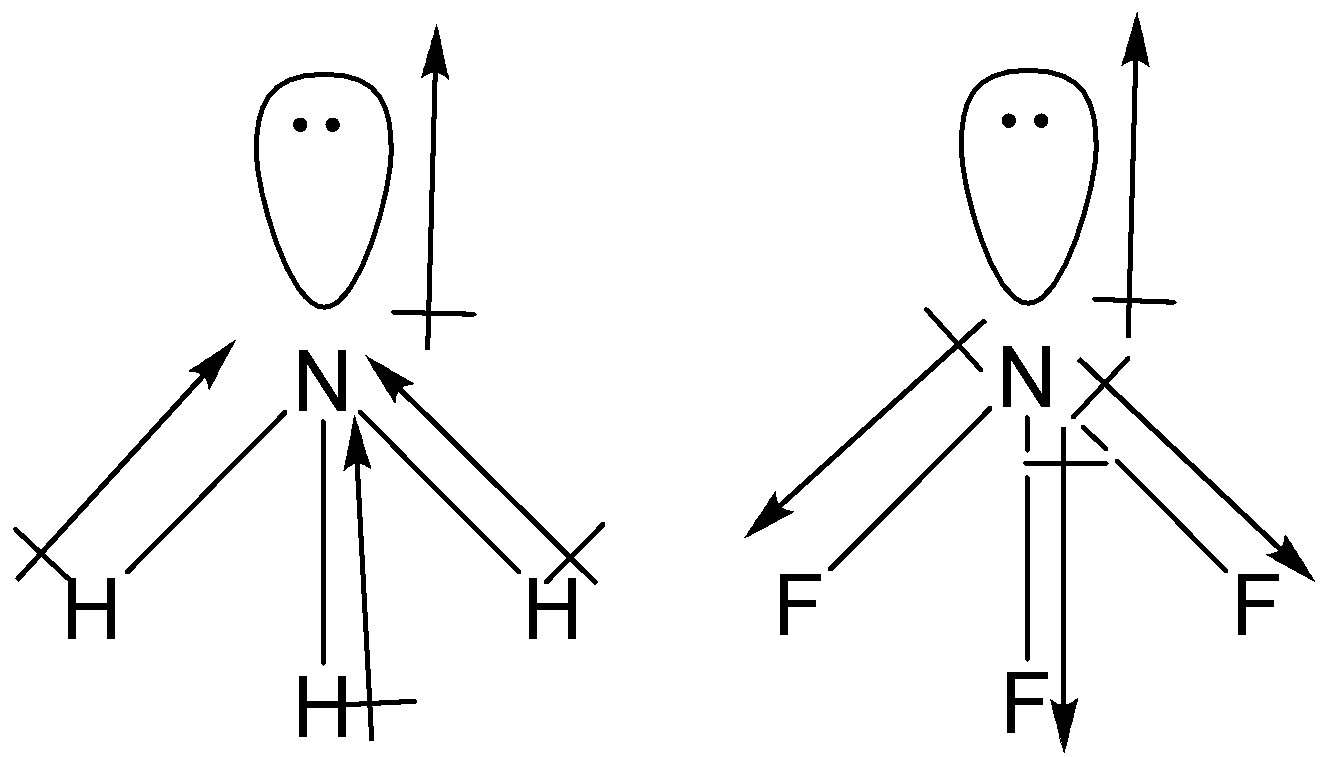

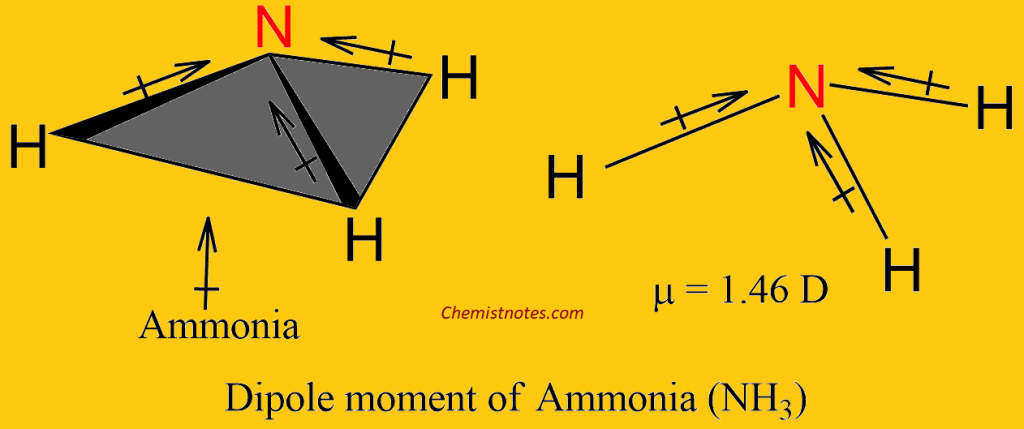




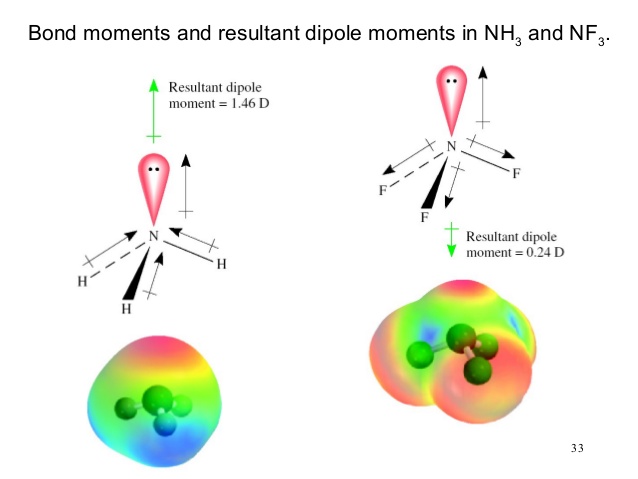
![SOLVED] The correct order of dipole moment is CH4<NF3<NH3<H2O - Self Study 365 SOLVED] The correct order of dipole moment is CH4<NF3<NH3<H2O - Self Study 365](https://static.tllms.com/moodle-migration/15617_9a10c2f086a16e947dad40a0c7d39c2ec62a11be_nhna.png)

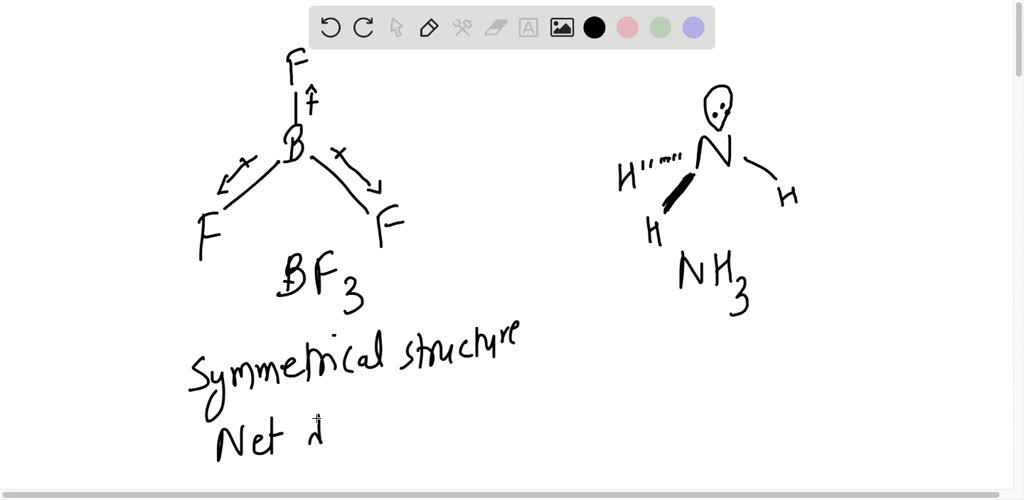


![Which out of \\[N{H_3}\\] and $N{F_3}$ has a higher dipole moment and why? Which out of \\[N{H_3}\\] and $N{F_3}$ has a higher dipole moment and why?](https://www.vedantu.com/question-sets/a32afb56-fc67-43ca-8a5c-e06f6fe2b15f5994708008049533229.png)
![Solved D) Has larger dipole moment (NH3, Or NF3] NH3 has | Chegg.com Solved D) Has larger dipole moment (NH3, Or NF3] NH3 has | Chegg.com](https://media.cheggcdn.com/study/a4f/a4f8fe7f-ba32-4d59-a27f-a1f5fd47c576/image.png)